How Cold Is Propane Liquid
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC proper noun Propane[1] | |||
| Systematic IUPAC name Tricarbane (never recommended[ane]) | |||
| Identifiers | |||
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| Beilstein Reference | 1730718 | ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.000.753 | ||
| EC Number |
| ||
| East number | E944 (glazing agents, ...) | ||
| Gmelin Reference | 25044 | ||
| KEGG |
| ||
| PubChem CID |
| ||
| RTECS number |
| ||
| UNII |
| ||
| United nations number | 1978 | ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Backdrop[3] | |||
| Chemical formula | C 3 H 8 | ||
| Tooth mass | 44.097 yard·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | two.0098 kg/miii (at 0 °C, 101.3 kPa) | ||
| Melting signal | −187.seven °C; −305.eight °F; 85.5 1000 | ||
| Boiling point | −42.25 to −42.04 °C; −44.05 to −43.67 °F; 230.xc to 231.xi K | ||
| Solubility in water | 47 mg⋅L−ane (at 0 °C) | ||
| log P | ii.236 | ||
| Vapor pressure level | 853.xvi kPa (at 21.1 °C (lxx.0 °F)) | ||
| Henry's law | 15 nmol⋅Pa−one⋅kg−one | ||
| Conjugate acid | Propanium | ||
| Magnetic susceptibility (χ) | −xl.5 × 10−half dozen cm3/mol | ||
| Dipole moment | 0.083 D[2] | ||
| Thermochemistry | |||
| Heat chapters (C) | 73.60 J⋅K−i⋅mol−i | ||
| Std enthalpy of | −105.2–104.ii kJ⋅mol−one | ||
| Std enthalpy of | −2.2197–ii.2187 MJ⋅mol−one | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |   | ||
| Signal word | Danger | ||
| Chance statements | H220 | ||
| Precautionary statements | P210 | ||
| NFPA 704 (fire diamond) | ii 4 0 | ||
| Flash point | −104 °C (−155 °F; 169 G) | ||
| Autoignition | 470 °C (878 °F; 743 K) | ||
| Explosive limits | 2.37–9.five% | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA one,000 ppm (one,800 mg/m3)[four] | ||
| REL (Recommended) | TWA 1,000 ppm (1,800 mg/chiliad3)[4] | ||
| IDLH (Immediate danger) | 2,100 ppm[4] | ||
| Related compounds | |||
| Related alkanes |
| ||
| Related compounds |
| ||
| Supplementary data page | |||
| Propane (data folio) | |||
| Except where otherwise noted, data are given for materials in their standard land (at 25 °C [77 °F], 100 kPa). Infobox references | |||

A 20 lb (9.1 kg) steel propane cylinder. This cylinder is fitted with an overfill prevention device (OPD) valve, as evidenced by the trilobular handwheel.
Propane () is a three-carbon alkane series with the molecular formula C3H8 . It is a gas at standard temperature and pressure level, but compressible to a transportable liquid. A by-production of natural gas processing and petroleum refining, it is commonly used as a fuel in domestic and industrial applications and in depression-emissions public transportation. Discovered in 1857 by the French pharmacist Marcellin Berthelot, it became commercially available in the Usa by 1911. Propane is ane of a grouping of liquefied petroleum gases (LP gases). The others include butane, propylene, butadiene, butylene, isobutylene, and mixtures thereof. Propane has lower volumetric energy density, but higher gravimetric energy density and burns more than cleanly than gasoline and coal.[6]
Propane gas has go a popular choice for barbecues and portable stoves because its low -42 °C boiling indicate makes it vaporise inside pressurised liquid containers (2 phases). Propane powers buses, forklifts, taxis, outboard boat motors, and ice resurfacing machines and is used for heat and cooking in recreational vehicles and campers.
History [edit]
Propane was discovered past the French chemist Marcellin Berthelot in 1857.[vii] It was found dissolved in Pennsylvanian light crude oil by Edmund Ronalds in 1864.[eight] [nine] Walter O. Snelling of the U.S. Bureau of Mines highlighted it equally a volatile component in gasoline in 1910, which was the kickoff of the propane manufacture in the United States. The volatility of these lighter hydrocarbons caused them to be known every bit "wild" considering of the high vapor pressures of unrefined gasoline. On March 31, 1912, The New York Times reported on Snelling's work with liquefied gas, saying "a steel bottle will comport enough gas to lite an ordinary habitation for three weeks".[x]
It was during this fourth dimension that Snelling, in cooperation with Frank P. Peterson, Chester Kerr, and Arthur Kerr, developed ways to liquefy the LP gases during the refining of gasoline. Together, they established American Gasol Co., the commencement commercial marketer of propane. Snelling had produced relatively pure propane by 1911, and on March 25, 1913, his method of processing and producing LP gases was issued patent #i,056,845.[11] A separate method of producing LP gas through compression was developed by Frank Peterson and its patent granted on July ii, 1912.[12]
The 1920s saw increased product of LP gas, with the first twelvemonth of recorded production totaling 223,000 US gallons (840 one thousand3) in 1922. In 1927, annual marketed LP gas production reached 1 1000000 United states gallons (iii,800 m3), and by 1935, the annual sales of LP gas had reached 56 1000000 The states gallons (210,000 miii). Major industry developments in the 1930s included the introduction of railroad tank motorcar transport, gas odorization, and the structure of local bottle-filling plants. The year 1945 marked the offset year that annual LP gas sales reached a billion gallons. By 1947, 62% of all U.South. homes had been equipped with either natural gas or propane for cooking.[11]
In 1950, 1,000 propane-fueled buses were ordered by the Chicago Transit Potency, and by 1958, sales in the U.S. had reached 7 billion U.s. gallons (26,000,000 thou3) annually. In 2004, it was reported to be a growing $8-billion to $ten-billion manufacture with over 15 billion Usa gallons (57,000,000 m3) of propane being used annually in the U.S.[13]
The "prop-" root establish in "propane" and names of other compounds with 3-carbon chains was derived from "propionic acrid",[14] which in turn was named later the Greek words protos (meaning start) and pion (fat).
During the COVID-19 pandemic, propane shortages were reported in the United States.[xv] [sixteen] [17]
Sources [edit]
Propane is produced every bit a by-production of two other processes, natural gas processing and petroleum refining. The processing of natural gas involves removal of butane, propane, and large amounts of ethane from the raw gas, to prevent condensation of these volatiles in natural gas pipelines. Additionally, oil refineries produce some propane as a past-product of bang-up petroleum into gasoline or heating oil.
The supply of propane cannot easily exist adjusted to meet increased need, considering of the past-product nature of propane production. Nearly xc% of U.S. propane is domestically produced.[18] The United states of america imports about 10% of the propane consumed each yr, with well-nigh 70% of that coming from Canada via pipeline and rails. The remaining thirty% of imported propane comes to the United States from other sources via ocean transport.
After it is separated from the crude oil, North American propane is stored in huge salt caverns. Examples of these are Fort Saskatchewan, Alberta; Mont Belvieu, Texas; and Conway, Kansas. These salt caverns[19] tin can store lxxx,000,000 barrels (xiii,000,000 miii) of propane.
Properties and reactions [edit]
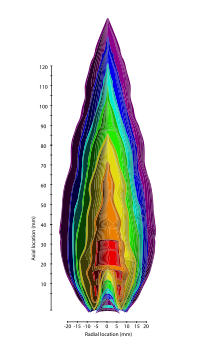
Pyrometry of a propane flame using sparse-filament velocimetry. The hottest parts of the flame are in a hollow cone-shaped area near its base of operations and pointing upward.
>i,750 1000 (1,480 °C)
i,700 K (ane,430 °C)
1,600 K (1,330 °C)
1,350 K (1,080 °C)
ane,100 Thou (830 °C)
875 Chiliad (602 °C)
750 Thou (477 °C)
Propane is a colorless, odorless gas. At normal pressure level it liquifies below its boiling indicate at −42 °C and solidifies below its melting point at −187.7 °C. Propane crystallizes in the infinite group P21/n.[20] [21] The low spacefilling of 58.five% (at ninety K), due to the bad stacking properties of the molecule, is the reason for the particularly low melting point.
Propane undergoes combustion reactions in a like style to other alkanes. In the presence of excess oxygen, propane burns to form water and carbon dioxide.
When insufficient oxygen is present for complete combustion, carbon monoxide, soot (carbon), or both, are formed as well:
Complete combustion of propane produces about 50 MJ/kg of heat.[22]
Propane combustion is much cleaner than that of coal or unleaded gasoline. Propane's per-BTU product of CO2 is most as low as that of natural gas.[23] Propane burns hotter than home heating oil or diesel fuel considering of the very high hydrogen content. The presence of C–C bonds, plus the multiple bonds of propylene and butylene, produce organic exhausts too carbon dioxide and water vapor during typical combustion. These bonds too cause propane to burn with a visible flame.
Free energy content [edit]
The enthalpy of combustion of propane gas where all products render to standard state, for example where water returns to its liquid state at standard temperature (known equally higher heating value), is (2,219.two ± 0.5) kJ/mol, or (50.33 ± 0.01) MJ/kg.[22]
The enthalpy of combustion of propane gas where products do not render to standard state, for example where the hot gases including water vapor get out a chimney, (known as lower heating value) is −2043.455 kJ/mol.[24] The lower estrus value is the amount of heat bachelor from burning the substance where the combustion products are vented to the atmosphere; for case, the oestrus from a fireplace when the flue is open up.
Density [edit]
The density of propane gas at 25 °C (77 °F) is ane.808 kg/m3, near 1.5x the density of air at the same temperature. The density of liquid propane at 25 °C (77 °F) is 0.493 thousand/cmiii, which is equivalent to 4.11 pounds per U.South. liquid gallon or 493 g/L. Propane expands at one.5% per 10 °F. Thus, liquid propane has a density of approximately 4.2 pounds per gallon (504 g/L) at sixty °F (15.half dozen °C).[25]
As the density of propane changes with temperature, this fact must exist considered every time when the awarding is connected with safety or custody transfer operations.[26]
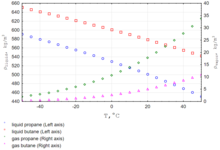
Temperature-Density curve for liquid/vapor propane
Uses [edit]
Portable stoves [edit]
Propane is a popular selection for barbecues and portable stoves because the low boiling indicate of −42 °C (−44 °F) makes information technology vaporize equally soon as it is released from its pressurized container. Therefore, no carburetor or other vaporizing device is required; a uncomplicated metering nozzle suffices.
Refrigerant [edit]
Blends of pure, dry out "isopropane" (R-290a) (isobutane/propane mixtures) and isobutane (R-600a) can be used as the circulating refrigerant in suitably constructed compressor-based refrigeration. Compared to fluorocarbons, propane has a negligible ozone depletion potential and very depression global warming potential (having a value of only three.3 times the GWP of carbon dioxide) and can serve every bit a functional replacement for R-12, R-22, R-134a, and other chlorofluorocarbon or hydrofluorocarbon refrigerants in conventional stationary refrigeration and air conditioning systems.[27] Because its global warming upshot is far less than current refrigerants, propane was called equally one of 5 replacement refrigerants approved by the EPA in 2015, for employ in systems specially designed to handle its flammability.[28]
Such substitution is widely prohibited or discouraged in motor vehicle air conditioning systems, on the grounds that using flammable hydrocarbons in systems originally designed to carry non-combustible refrigerant presents a significant take a chance of fire or explosion.[29]
Vendors and advocates of hydrocarbon refrigerants fence confronting such bans on the grounds that at that place have been very few such incidents relative to the number of vehicle air conditioning systems filled with hydrocarbons.[30] [31]
Propane is also instrumental in providing off-the-filigree refrigeration, as the free energy source for a gas absorption refrigerator and is normally used for camping and recreational vehicles.
Domestic and industrial fuel [edit]

Domestic spherical steel pressure vessel for propane storage.
Since information technology can be transported easily, information technology is a pop fuel for dwelling oestrus and backup electric generation in sparsely populated areas that do not have natural gas pipelines.
In rural areas of North America, as well as northern Australia, propane is used to heat livestock facilities, in grain dryers, and other heat-producing appliances. When used for heating or grain drying it is usually stored in a large, permanently-placed cylinder which is refilled by a propane-commitment truck. As of 2014[update], 6.2 million American households apply propane equally their primary heating fuel.[18]
In North America, local delivery trucks with an average cylinder size of 3,000 The states gallons (11 gthree), make full upward large cylinders that are permanently installed on the property, or other service trucks exchange empty cylinders of propane with filled cylinders. Big tractor-trailer trucks, with an average cylinder size of x,000 United states of america gallons (38 m3), transport propane from the pipeline or refinery to the local majority establish. The bobtail tank truck is non unique to the North American market place, though the practice is non every bit common elsewhere, and the vehicles are generally called tankers. In many countries, propane is delivered to end-users via minor or medium-sized individual cylinders, while empty cylinders are removed for refilling at a key location.
At that place are also customs propane systems, with a primal cylinder feeding individual homes.[32]
Motor fuel [edit]
In the U.S., over 190,000 on-road vehicles use propane, and over 450,000 forklifts utilise information technology for power. It is the 3rd near popular vehicle fuel in the earth,[33] behind gasoline and diesel fuel. In other parts of the world, propane used in vehicles is known equally autogas. In 2007, approximately 13 one thousand thousand vehicles worldwide use autogas.[33]
The advantage of propane in cars is its liquid state at a moderate force per unit area. This allows fast refill times, affordable fuel cylinder structure, and price ranges typically just over half that of gasoline. Meanwhile, it is noticeably cleaner (both in treatment, and in combustion), results in less engine wear (due to carbon deposits) without diluting engine oil (frequently extending oil-change intervals), and until recently[ when? ] was relatively low-toll in Due north America. The octane rating of propane is relatively loftier at 110. In the The states the propane fueling infrastructure is the most adult of all culling vehicle fuels. Many converted vehicles have provisions for topping off from "barbecue bottles". Purpose-congenital vehicles are oft in commercially endemic fleets, and accept private fueling facilities. A farther saving for propane fuel vehicle operators, especially in fleets, is that theft is much more than difficult than with gasoline or diesel fuels.
Propane is also used as fuel for small engines, peculiarly those used indoors or in areas with insufficient fresh air and ventilation to carry away the more toxic frazzle of an engine running on gasoline or diesel. More recently,[ when? ] there have been backyard-care products like string trimmers, lawn mowers and leafage blowers intended for outdoor use, but fueled by propane in society to reduce air pollution.[34]
Many heavy-duty highway trucks utilise propane as a boost, where information technology is added through the turbocharger, to mix with diesel fuel droplets. Propane droplets' very high hydrogen content helps the diesel fuel to burn down hotter and therefore more completely. This provides more torque, more horsepower, and a cleaner frazzle for the trucks. It is normal for a 7-liter medium-duty diesel truck engine to increase fuel economy past 20 to 33 pct when a propane boost system is used. It is cheaper considering propane is much cheaper than diesel fuel. The longer distance a cross-country trucker can travel on a total load of combined diesel fuel and propane fuel ways they can maintain federal hours of work rules with 2 fewer fuel stops in a cross-country trip. Truckers, tractor pulling competitions, and farmers have been using a propane boost system for over forty years[ when? ] in North America.
Shipping fuel [edit]
International ships can reuse propane from ocean-going ships that transport LPG because equally the sun evaporates the propane during the voyage, the international send catches the evaporating propane gas and feeds it into the air intake organisation of the send'due south diesel engines. This reduces bunker fuel consumption and the pollution produced by the ships. There is an international agreement to apply either propane or CNG as a mandatory condiment to the bunker fuel for all body of water traveling ships showtime in 2020.
Propane is generally stored and transported in steel cylinders as a liquid with a vapor space above the liquid. The vapor pressure in the cylinder is a part of temperature. When gaseous propane is drawn at a high rate, the latent heat of vaporization required to produce the gas volition crusade the canteen to cool. (This is why water often condenses on the sides of the bottle and and so freezes). Since lightweight, loftier-octane propane vaporizes before the heavier, low-octane propane, the ignition properties change every bit the cylinder empties. For these reasons, the liquid is ofttimes withdrawn using a dip tube.
Other uses [edit]
- Propane is the primary flammable gas in blowtorches for soldering.
- Propane is used in oxy-fuel welding and cutting. Propane does not fire as hot every bit acetylene in its inner cone, and so it is rarely used for welding. Propane, however, has a very high number of BTUs per cubic human foot in its outer cone, then with the right torch (injector way) information technology tin make a faster and cleaner cut than acetylene, and is much more useful for heating and bending than acetylene.
- Propane is used as a feedstock for the product of base of operations petrochemicals in steam cracking.
- Propane is the chief fuel for gasbag balloons.
- It is used in semiconductor manufacture to deposit silicon carbide.
- Propane is ordinarily used in theme parks and in flick production as an inexpensive, high-energy fuel for explosions and other special furnishings.
- Propane is used as a propellant, relying on the expansion of the gas to fire the projectile. It does not ignite the gas. The use of a liquefied gas gives more shots per cylinder, compared to a compressed gas.
- Propane is as well used as a cooking fuel.
- Propane is used every bit a propellant for many household aerosol sprays, including shaving creams and air fresheners.
- Propane is a promising feedstock for the production of propylene[35] [36] and acrylic acid.[37] [38] [39] [40]
Liquified propane is used in the extraction of creature fats and vegetable oils.[41]
Purity [edit]
The N American standard grade of automotive-use propane is rated Hard disk-5 (Heavy Duty 5%). HD-five grade has a maximum of 5 per centum butane, but propane sold in Europe has a maximum allowable corporeality of butane of 30 per centum, significant it is not the same fuel as Hd-5. The LPG used as auto fuel and cooking gas in Asia and Australia too has very high butane content.
Propylene (also chosen propene) can exist a contaminant of commercial propane. Propane containing as well much propene is not suited for about vehicle fuels. Hard disk-five is a specification that establishes a maximum concentration of v% propene in propane. Propane and other LP gas specifications are established in ASTM D-1835.[42] All propane fuels include an odorant, well-nigh always ethanethiol, so that the gas tin be smelled easily in example of a leak. Propane every bit Hard disk-5 was originally intended for apply as vehicle fuel. HD-v is currently beingness used in all propane applications.
Typically in the Us and Canada, LPG is primarily propane (at to the lowest degree xc%), while the rest is more often than not ethane, propylene, butane, and odorants including ethyl mercaptan.[43] [44] This is the HD-5 standard, (maximum allowable propylene content, and no more 5% butanes and ethane) defined past the American Society for Testing and Materials by its Standard 1835 for internal combustion engines. Not all products labeled "LPG" conform to this standard, even so. In Mexico, for example, gas labeled "LPG" may consist of lx% propane and twoscore% butane. "The exact proportion of this combination varies by country, depending on international prices, on the availability of components and, peculiarly, on the climatic weather condition that favor LPG with college butane content in warmer regions and propane in cold areas".[45]
Comparison with natural gas [edit]
Propane is bought and stored in a liquid form, LPG. It can easily exist stored in a relatively pocket-size space.
By comparing, compressed natural gas (CNG) cannot be liquefied by pinch at normal temperatures, as these are well above its critical temperature. Equally a gas, very high pressure is required to store useful quantities. This poses the risk that, in an accident, just as with any compressed gas cylinder (such as a CO2 cylinder used for a soda concession) a CNG cylinder may burst with nifty force, or leak rapidly plenty to become a self-propelled missile. Therefore, CNG is much less efficient to store than propane, due to the big cylinder volume required. An alternative means of storing natural gas is as a cryogenic liquid in an insulated container as liquefied natural gas (LNG). This grade of storage is at low pressure level and is effectually 3.v times every bit efficient every bit storing it as CNG.
Unlike propane, if a spill occurs, CNG volition evaporate and dissipate because information technology is lighter than air.
Propane is much more ordinarily used to fuel vehicles than is natural gas, because that equipment costs less. Propane requires just 1,220 kilopascals (177 psi) of pressure to keep it liquid at 37.viii °C (100 °F).[46]
Hazards [edit]
Propane is a uncomplicated asphyxiant.[47] Unlike natural gas, propane is denser than air. It may accumulate in low spaces and virtually the floor. When driveling as an inhalant, it may cause hypoxia (lack of oxygen), pneumonia, cardiac failure or cardiac arrest.[48] [49] Propane has low toxicity since it is not readily captivated and is not biologically agile. Commonly stored under pressure at room temperature, propane and its mixtures volition flash evaporate at atmospheric pressure and absurd well below the freezing point of water. The cold gas, which appears white due to moisture condensing from the air, may cause frostbite.
Propane is denser than air. If a leak in a propane fuel arrangement occurs, the vaporized gas will have a tendency to sink into any enclosed surface area and thus poses a run a risk of explosion and fire. The typical scenario is a leaking cylinder stored in a basement; the propane leak drifts across the floor to the airplane pilot light on the furnace or h2o heater, and results in an explosion or fire. This property makes propane generally unsuitable as a fuel for boats. In 2007, a heavily investigated vapor-related explosion occurred in Ghent, West Virginia, U.S., killing four people and completely destroying the Niggling General convenience store on Flat Tiptop Road, causing several injuries.[50] [51]
Some other take chances associated with propane storage and transport is known every bit a BLEVE or boiling liquid expanding vapor explosion. The Kingman Explosion involved a railroad tank car in Kingman, Arizona, U.S., in 1973 during a propane transfer. The burn down and subsequent explosions resulted in twelve fatalities and numerous injuries.[52]
Retail cost [edit]
The states [edit]
Equally of October 2013[update], the retail toll of propane was approximately $2.37 per gallon, or roughly $25.95 per ane 1000000 BTUs.[53] This means that filling a 500-gallon propane tank, which is what households that use propane equally their main source of free energy usually require, costs $948 (eighty% of 500 gallons or 400 gallons), a 7.5% increase on the 2012–2013 winter season boilerplate US price.[54] However, propane costs per gallon change significantly from one state to another: the Energy Information Administration (EIA) quotes a $2.995 per gallon average on the East Coast for October 2013,[55] while the figure for the Midwest was $i.860 for the same period.[56]
Every bit of December 2015[update] the propane retail cost was approximately $1.97 per gallon.[57] This ways that filling a 500-gallon propane tank to 80% chapters costs $788, a 16.9% decrease or $160 less from the Nov 2013 quote in this section. Similar regional differences in prices are present with the December 2015 EIA effigy for the Due east Coast at $2.67 per gallon and the Midwest at $one.43 per gallon.[57]
Every bit of August 2018[update] the average The states propane retail cost was approximately $2.48 per gallon. The wholesale price of propane in the U.South. e'er drops in the summer every bit virtually homes do non require it for home heating. The wholesale price of propane in the summer of 2018 was between 86 cents to 96 cents per U.S. gallon, based on a truckload or railway car load. The cost for home heating is exactly double that cost; at 95 cents per gallon wholesale, a habitation-delivered price was $ane.90 per gallon if ordered 500 gallons at a time. Prices in the midwest are always cheaper than California. Prices for abode delivery always get up nearly the end of August or the starting time few days of September when people start ordering their home tanks to be filled.[58]
Run into also [edit]
- Blau gas
- National Propane Gas Association
- Hank Colina
References [edit]
- ^ a b "Front end Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Imperial Society of Chemistry. 2014. p. 4. doi:x.1039/9781849733069-FP001. ISBN978-0-85404-182-4.
Similarly, the retained names 'ethane', 'propane', and 'butane' were never replaced by systematic names 'dicarbane', 'tricarbane', and 'tetracarbane' equally recommended for analogues of silane, 'disilane'; phosphane, 'triphosphane'; and sulfane, 'tetrasulfane'.
- ^ Lide, David R. Jr. (1960). "Microwave Spectrum, Structure, and Dipole Moment of Propane". J. Chem. Phys. 33 (5): 1514–1518. Bibcode:1960JChPh..33.1514L. doi:10.1063/ane.1731434.
- ^ Record of Propane in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b c NIOSH Pocket Guide to Chemic Hazards. "#0524". National Institute for Occupational Safety and Health (NIOSH).
- ^ GOV, NOAA Office of Response and Restoration, U.s.a.. "PROPANE – CAMEO Chemicals – NOAA". cameochemicals.noaa.gov.
- ^ "Fuels". www.globalfueleconomy.org . Retrieved 2022-04-12 .
- ^ (France), Académie des Sciences (1905). "Comptes rendus hebdomadaires des séances de 50'Académie des sciences" (in French). 140.
- ^ Roscoe, H.Eastward.; Schorlemmer, C. (1881). Treatise on Chemical science. Vol. iii. Macmillan. pp. 144–145.
- ^ Watts, H. (1868). Dictionary of Chemistry. Vol. 4. p. 385.
- ^ "GAS Institute IN STEEL Bottle.; Dr. Snelling's Process Gives Month's Supply in Liquid Form". The New York Times. April 1, 1912. p. 9. Retrieved 2007-12-22 .
- ^ a b National Propane Gas Association. "The History of Propane". Archived from the original on January 11, 2011. Retrieved 2007-12-22 .
{{cite spider web}}: CS1 maint: unfit URL (link) - ^ "The Kickoff Fifty Years of LP-Gas: An Industry Chronology" (PDF). LPGA Times. January 1962. Archived from the original (PDF) on 2006-10-07. , Folio 17.
- ^ Propane Education & Research Council. "Fact Sheet – The History of Propane". Archived from the original on February 16, 2004. Retrieved 2007-12-22 .
{{cite web}}: CS1 maint: unfit URL (link) - ^ "Online Etymology Lexicon entry for propane". Etymonline.com. Retrieved 2010-10-29 .
- ^ Puente, Victor. "Propane shortage: An unexpected side effect of the pandemic and restaurant mandates". WKYT . Retrieved 2021-01-30 .
{{cite web}}: CS1 maint: url-condition (link) - ^ Lott, Jennifer. "Southwest Louisiana is experiencing a propane supply shortage". KPLC . Retrieved 2021-01-thirty .
{{cite web}}: CS1 maint: url-status (link) - ^ Peguero, Joshua. "Pandemic is creating an increase in demand for propane, as some homeowners struggle to get some". WBAY . Retrieved 2021-01-30 .
{{cite web}}: CS1 maint: url-condition (link) - ^ a b Sloan, Michael. "2016 Propane Market place Outlook" (PDF). Propane Educational activity and Research Council. Retrieved 19 January 2018.
- ^ Argonne National Laborator (1999). "Salt Cavern Information Center". Archived from the original on 2007-12-23. Retrieved 2007-12-22 .
- ^ "geometry of crystalline propane".
- ^ Boese R, Weiss HC, Blaser D (1999). "The melting point alternation in the curt-chain n-alkanes: Single-crystal X-ray analyses of propane at 30 K and of due north-butane to northward-nonane at 90 Chiliad". Angew Chem Int Ed. 38: 988–992. doi:10.1002/(SICI)1521-3773(19990401)38:vii<988::AID-ANIE988>3.3.CO;ii-S.
- ^ a b Propane. NIST Standard Reference Data referring to Pittam, D. A.; Pilcher, G. (1972). "Measurements of heats of combustion by flame calorimetry. Function 8.—Methane, ethane, propane, n-butane and 2-methylpropane". Periodical of the Chemic Social club, Faraday Transactions ane: Physical Chemistry in Condensed Phases. 68: 2224. doi:ten.1039/f19726802224. and Rossini, F.D. (1934). "Calorimetric determination of the heats of combustion of ethane, propane, normal butane, and normal pentane". Bureau of Standards Journal of Research. 12 (6): 735–750. doi:10.6028/jres.012.059.
- ^ United States Free energy Information Clan. "How much carbon dioxide is produced when different fuels are burned". Retrieved 2019-03-25 .
- ^ Ҫengel, Yunus A.; Boles, Michael A. (2006). Thermodynamics: An Engineering science Approach (5th ed.). McGrawHill. p. 925. ISBN978-0-07-288495-1.
- ^ Razmi, Amir (May 2019). "Propylene Product past Propane Dehydrogenation (PDH)". Engineering: 3.
- ^ Zivenko, Oleksiy (2019). "LPG Accounting Specificity During ITS Storage and Transportation". Measuring Equipment and Metrology. 80 (iii): 21–27. doi:10.23939/istcmtm2019.03.021. ISSN 0368-6418. S2CID 211776025.
- ^ "European Committee on retrofit refrigerants for stationary applications" (PDF). Archived from the original on August 5, 2009. Retrieved 2010-x-29 .
{{cite spider web}}: CS1 maint: unfit URL (link) - ^ Koch, Wendy (March 6, 2015). "Why Your Refrigerator Pollutes and How It'south Changing". National Geographic. Retrieved 22 December 2021.
- ^ "U.S. EPA hydrocarbon-refrigerants FAQ". Epa.gov. Retrieved 2010-10-29 .
Compendium of hydrocarbon-refrigerant policy statements, October 2006. vasa.org.au
"MACS bulletin: hydrocarbon refrigerant usage in vehicles" (PDF). Archived from the original (PDF) on 2011-01-05. Retrieved 2010-10-29 .
"Society of Automotive Enginers hydrocarbon refrigerant message". Sae.org. 2005-04-27. Archived from the original on 2005-05-05. Retrieved 2010-10-29 .
"Shade Tree Mechanic on hydrocarbon refrigerants". Shadetreemechanic.com. 2005-04-27. Retrieved 2010-x-29 .
"Saskatchewan Labour bulletin on hydrocarbon refrigerants in vehicles". Labour.gov.sk.ca. 2010-06-29. Archived from the original on 2009-07-01. Retrieved 2010-ten-29 .
VASA on refrigerant legality & advisability. vasa.org.au
"Queensland (Australia) authorities alarm on hydrocarbon refrigerants" (PDF). Energy.qld.gov.au. Archived from the original on December 17, 2008. Retrieved 2010-x-29 .
{{cite web}}: CS1 maint: unfit URL (link) - ^ "New South Wales (Australia) Parliamentary tape, 16 Oct 1997". Parliament.nsw.gov.au. 1997-ten-16. Archived from the original on ane July 2009. Retrieved 2010-ten-29 .
- ^ "New South Wales (Australia) Parliamentary record, 29 June 2000". Parliament.nsw.gov.au. Archived from the original on 22 May 2005. Retrieved 2010-10-29 .
- ^ Quango, Propane Didactics & Inquiry. "Community Propane Systems | Propane.com". Propane . Retrieved 2021-12-28 .
- ^ a b Propane Education & Research Council. "Autogas". PERC. Archived from the original on September 23, 2010. Retrieved 2012-05-17 .
{{cite web}}: CS1 maint: unfit URL (link) - ^ "Facts Nearly Propane: America's Exceptional Energy" (PDF). National Propane Gas Clan. Apr 2001. Archived from the original (PDF) on December 21, 2016. Retrieved December 15, 2016.
- ^ Hernández, Juan Pablo; Echavarría, Adriana; Palacio, Luz Amparo (2013). "Synthesis of two new Nickel and Copper- Nickel vanadates used for propane oxidative dehydrogenation". Revista Facultad de Ingeniería Universidad de Antioquia (67): 137–145. ISSN 0120-6230.
- ^ Zea, Hugo; Figueiredo, Jose 50.; Carballo, Luis (2011). "PROMOTING Effect OF Mo ON Pd / g-Al2O3 SUPPORTED CATALYSTS IN THE OXIDATIVE DEHYDROGENATION OF PROPANE". DYNA. 78 (170): 159–166. ISSN 0012-7353.
- ^ Naumann d'Alnoncourt, Raoul; Csepei, Lénárd-István; Hävecker, Michael; Girgsdies, Frank; Schuster, Manfred Due east; Schlögl, Robert; Trunschke, Annette (2014). "The reaction network in propane oxidation over phase-pure MoVTeNb M1 oxide catalysts" (PDF). Journal of Catalysis. 311: 369–385. doi:10.1016/j.jcat.2013.12.008. hdl:11858/00-001M-0000-0014-F434-5. Archived from the original (PDF) on 2016-02-15. Retrieved 2017-12-23 .
- ^ Amakawa, Kazuhiko; Kolen'Ko, Yury V; Villa, Alberto; Schuster, Manfred E/; Csepei, Lénárd-István; Weinberg, Gisela; Wrabetz, Sabine; Naumann d'Alnoncourt, Raoul; Girgsdies, Frank; Prati, Laura; Schlögl, Robert; Trunschke, Annette (2013). "Multifunctionality of Crystalline MoV(TeNb) M1 Oxide Catalysts in Selective Oxidation of Propane and Benzyl Alcohol". ACS Catalysis. 3 (6): 1103–1113. doi:10.1021/cs400010q.
- ^ Hävecker, Michael; Wrabetz, Sabine; Kröhnert, Jutta; Csepei, Lenard-Istvan; Naumann d'Alnoncourt, Raoul; Kolen'Ko, Yury V; Girgsdies, Frank; Schlögl, Robert; Trunschke, Annette (2012). "Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid" (PDF). Journal of Catalysis. 285: 48–threescore. doi:10.1016/j.jcat.2011.09.012. hdl:11858/00-001M-0000-0012-1BEB-F. Archived from the original (PDF) on 2016-10-thirty. Retrieved 2017-12-23 .
- ^ Kinetic studies of propane oxidation on Mo and V based mixed oxide catalysts (PDF). 2011.
- ^ Stoye, Dieter (2000). "Solvents". Ullmann's Encyclopedia of Industrial Chemical science. Weinheim: Wiley-VCH. doi:ten.1002/14356007.a24_437.
- ^ "ASTM D1835 - 16 Standard Specification for Liquefied Petroleum (LP) Gases". www.astm.org.
- ^ Amerigas. "Amerigas Material Safe Data Sheet for Odorized Propane" (PDF). Archived from the original (PDF) on 2011-12-09. Retrieved 2011-x-24 .
- ^ Suburban Propane. "Suburban Propane Material Safety Data Sail for Commercial Odorized Propane" (PDF). Archived from the original (PDF) on 2011-x-25. Retrieved 2011-10-24 .
- ^ Mexican Ministry building of Energy. "Liquefied Petroleum Gas Market Outlook 2008–2017" (PDF). Mexican Ministry of Energy. Archived from the original (PDF) on 2012-05-10. Retrieved 2012-05-17 .
- ^ "Propane Vapor Force per unit area". The Applied science ToolBox. 2005. Retrieved 2008-07-28 .
- ^ "Propane". The National Plant for Occupational Prophylactic and Health (NIOSH). Retrieved 2016-05-12 .
Propane is a unproblematic asphyxiant and does not present an IDLH hazard at concentrations below its lower explosive limit (LEL). The chosen IDLH is based on the LEL of 21,000 ppm rounded down to twenty,000 ppm.
- ^ "Inhalants – Facts and Statistics". Greater Dallas Quango on Alcohol & Drug Corruption. March four, 2006. Archived from the original on 2009-04-08.
- ^ "Inhalants". National Inhalant Prevention Coalition. 30 May 2020.
- ^ "Little General Store Propane Explosion". United states of america Chemical Safety and Hazard Investigation Lath. September 25, 2008. Retrieved June sixteen, 2021.
- ^ U.s. Chemical Safety and Take chances Investigation Board (September 25, 2008). "Investigation Report:Little General Store-Propane Explosion (iv killed, six injured)" (PDF) . Retrieved June 16, 2021.
- ^ "The Disaster Story". Kingman Historic Commune. Retrieved 1 July 2013.
- ^ U.s. Energy Data Administration (Nov 12, 2013). "Heating Oil and Propane Prices".
- ^ Propane Deal (Nov 12, 2013). "Current Propane Prices".
- ^ U.s.a. Energy Information Administration (November 12, 2013). "East Coast Heating Oil and Propane Prices".
- ^ U.s.a. Free energy Information Administration (November 12, 2013). "Midwest Heating Oil and Propane Prices".
- ^ a b Usa Energy Information Assistants (December 12, 2015). "Residential Propane: Weekly Heating Oil and Propane Prices (October – March)".
- ^ US Energy Information Administration (August eleven, 2018). "Residential Propane: Weekly Heating Oil and Propane Prices (October – March)".
External links [edit]
![]()
Wikimedia Commons has media related to Propane.
- Canadian Propane Association
- Kaoru Fujimoto; Hiroshi Kaneko; Qianwen Zhang; Qingjie Ge; Xiaohong Li (2007). "Direct synthesis of propane/butane from synthesis gas". In Noronha, F.B.; Schmal, Chiliad.; Sousa-Aguiar, E.F. (eds.). Natural Gas Conversion Eight, Proceedings of the 8th Natural Gas Conversion Symposium. Studies in Surface Science and Catalysis. Vol. 167. Elsevier. pp. 349–354. doi:10.1016/S0167-2991(07)80156-X. ISBN9780444530783. (syngas)
- International Chemic Safety Carte 0319
- National Propane Gas Association (U.S.)
- NIOSH Pocket Guide to Chemical Hazards
- Propane Education & Inquiry Council (U.Due south.)
- Propane Properties Explained Descriptive Breakdown of Propane Characteristics
- UKLPG: Propane and Butane in the UK
- US Energy Information Administration
- World LP Gas Clan (WLPGA)
How Cold Is Propane Liquid,
Source: https://en.wikipedia.org/wiki/Propane
Posted by: barkleymidess.blogspot.com







0 Response to "How Cold Is Propane Liquid"
Post a Comment